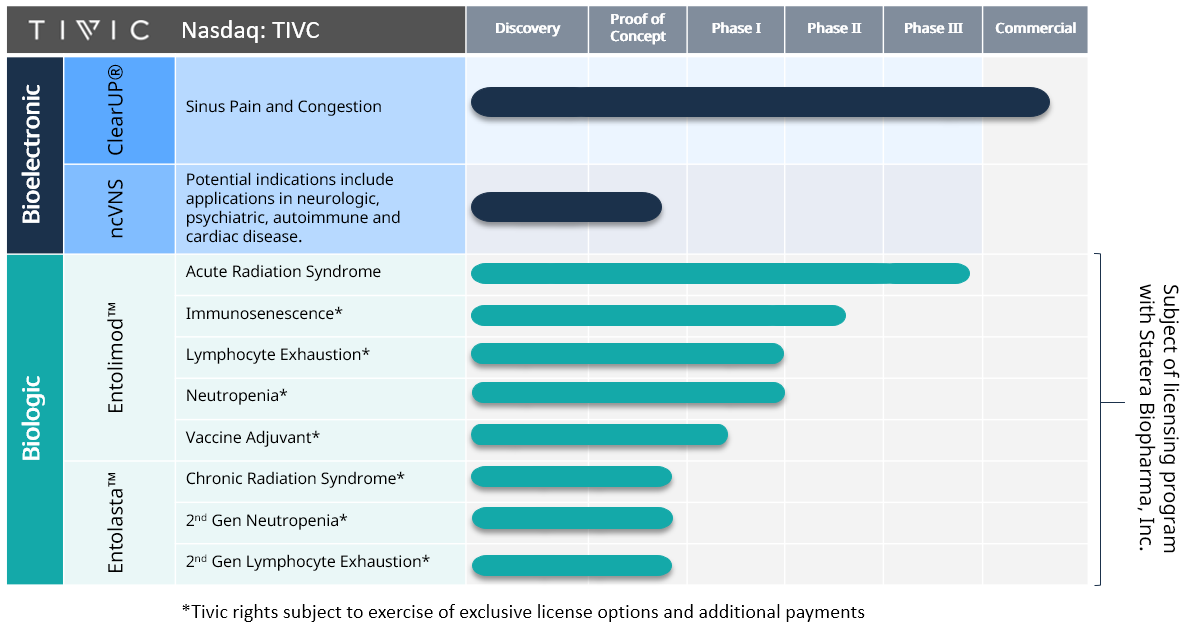
Pipeline
Entolimod™ as a radiation countermeasure for Acute Radiation Syndrome
The FDA has granted investigational new drug, fast-track, and orphan drug statuses to Entolimod™. Its safety, efficacy, and animal-to-human dose conversion data allowed its progression with a pre-emergency use authorization application submission.
This study was published in the peer-review journal Drug Discovery Today, Volume 26, Number 1, January 2021. Vijay K. Singh and Thomas M. Seed.
Tivic VNS Optimization Study
Tivic Health Study Demonstrated Clinically Effective Biological Changes in Response to Cervical Vagus Nerve Stimulation
Tivic Health’s pilot research study validated its novel and proprietary approach to ncVNS using objective measures of the autonomic nervous system, cardiac function, and brain activity. The strength of the data suggest Tivic’s differentiated approach to ncVNS may yield superior clinical outcomes when compared to the current status quo in vagus nerve stimulation.
Shubham Debnath1, 2, Fylaktis Fylaktou1, 2, Todd J. Levy1, 2, Blake T. Gurfein3, Theodoros P. Zanos1, 2
1 Institute of Bioelectronic Medicine, Feinstein Institutes for Medical Research, 350 Community
Dr, Manhasset, NY, 11030
2Institute of Health System Science, Feinstein Institutes for Medical Research, 350 Community Dr., Manhasset, NY 11030
3Tivic Health Systems, Inc., Fremont, CA 94025
The clinical trial showing the effectiveness of Tivic’s ClearUP® product technology was published in the peer-reviewed publication International Forum of Allergy & Rhinology and is titled
Microcurrent technology for rapid relief of sinus pain: a randomized, placebo‐controlled, double‐blinded clinical trial.
The study was published in the peer-reviewed publication International Forum of Allergy & Rhinology (Vol. 9, No. 4, pp. 352-356). Maul, X. A., Borchard, N. A., Hwang, P. H., & Nayak, J. V. (2019, April).
The study was published in the peer-reviewed journal Bioelectronic Medicine, 5(1), 1-9. Goldsobel, A. B., Prabhakar, N., & Gurfein, B. T. (2019).